Clinical Testing Guidance from CDC

Clinical Testing Guidance for Coccidioidomycosis, Histoplasmosis, and Blastomycosis in Patients with Community-Acquired Pneumonia
A number of the diseases that the Mycotic Diseases Branch at the CDC focuses on have pulmonary manifestations, and symptoms can be similar to other respiratory diseases, leading to missed or delayed diagnosis. In an effort to increase awareness and early diagnosis, these epidemiologists have developed clinical diagnostic algorithms for coccidioidomycosis, blastomycosis, and histoplasmosis.
In the Clinical Testing Guidance for Coccidioidomycosis, Histoplasmosis, and Blastomycosis in Patients with Community-Acquired Pneumonia guide, clinicians in urgent care and outpatient settings can use these algorithms to help make determinations about clinical testing for coccidioidomycosis, histoplasmosis, and blastomycosis in patients with community-acquired pneumonia (CAP). The algorithms address an important gap in guidelines for CAP diagnosis by providing recommendations for primary care, outpatient, and urgent care providers for diagnosing fungal pneumonias. Existing guidelines for CAP diagnosis focus on bacterial and viral CAP.
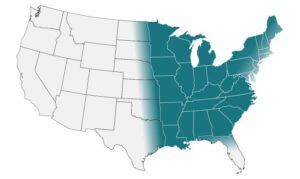
Find Community- Acquired Pneumonia (CAP): Clinical Testing Algorithm for Blastomycosis HERE.
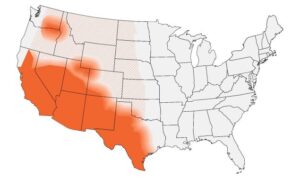
Find Community-Acquired Pneumonia (CAP): Clinical Testing Algorithm for Coccidioidomycosis HERE.
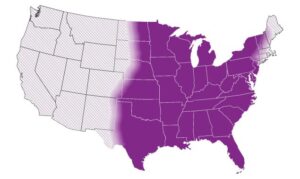
Find Community-Acquired Pneumonia (CAP): Clinical Testing Algorithm for Histoplasmosis HERE.
The Association of Pulmonary Advanced Practice Providers (APAPP) works to advance the profession of APPs in Pulmonary Medicine. We actively seek out opportunities and educational articles from trusted organizations like the CDC to help educate our members and ourselves on news in our field, with the goal of providing improved patient care.






